Tirzepatide
Treatment of obesity and overweight conditions
Potential application in reducing cardiovascular risk factors
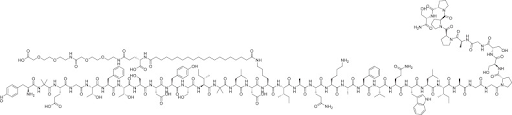
Tirzepatide is a synthetic peptide that functions as a dual agonist for glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. It is approved for the treatment of type 2 diabetes and obesity, marketed under the brand names Mounjaro and Zepbound, respectively.
Mechanism of action
Tirzepatide binds to GIP and GLP-1 receptors, enhancing insulin secretion, suppressing glucagon release, delaying gastric emptying, and promoting satiety. These combined actions lead to improved glycemic control and weight reduction. Its molecular modifications extend its half-life to approximately five days, allowing for once-weekly dosing.
Notable Studies
The SURPASS clinical trials demonstrated significant reductions in HbA1c and body weight in patients with type 2 diabetes treated with Tirzepatide.
The SURMOUNT-1 trial showed that Tirzepatide led to substantial weight loss in obese individuals without diabetes.
Risk Associated
Common side effects include nausea, vomiting, diarrhea, constipation, and abdominal discomfort. There is a potential risk of thyroid C-cell tumors, as observed in rodent studies; however, the relevance to humans is unknown. Tirzepatide is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.
Dosage
For type 2 diabetes: Initiate at 2.5 mg subcutaneously once weekly for 4 weeks, then increase to 5 mg once weekly. The dose may be increased in 2.5 mg increments after at least 4 weeks at the current dose, up to a maximum of 15 mg once weekly.
External link

Tirzepatide
Treatment of obesity and overweight conditions
Potential application in reducing cardiovascular risk factors
Mechanism of action
Tirzepatide binds to GIP and GLP-1 receptors, enhancing insulin secretion, suppressing glucagon release, delaying gastric emptying, and promoting satiety. These combined actions lead to improved glycemic control and weight reduction. Its molecular modifications extend its half-life to approximately five days, allowing for once-weekly dosing.
Risk
Associated
Common side effects include nausea, vomiting, diarrhea, constipation, and abdominal discomfort. There is a potential risk of thyroid C-cell tumors, as observed in rodent studies; however, the relevance to humans is unknown. Tirzepatide is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.
Notable Studies
The SURPASS clinical trials demonstrated significant reductions in HbA1c and body weight in patients with type 2 diabetes treated with Tirzepatide.
The SURMOUNT-1 trial showed that Tirzepatide led to substantial weight loss in obese individuals without diabetes.
Dosage
For type 2 diabetes: Initiate at 2.5 mg subcutaneously once weekly for 4 weeks, then increase to 5 mg once weekly. The dose may be increased in 2.5 mg increments after at least 4 weeks at the current dose, up to a maximum of 15 mg once weekly.
External link
Tirzepatide
Treatment of obesity and overweight conditions
Potential application in reducing cardiovascular risk factors
Mechanism of action
Tirzepatide binds to GIP and GLP-1 receptors, enhancing insulin secretion, suppressing glucagon release, delaying gastric emptying, and promoting satiety. These combined actions lead to improved glycemic control and weight reduction. Its molecular modifications extend its half-life to approximately five days, allowing for once-weekly dosing.

Risk
Associated
Common side effects include nausea, vomiting, diarrhea, constipation, and abdominal discomfort. There is a potential risk of thyroid C-cell tumors, as observed in rodent studies; however, the relevance to humans is unknown. Tirzepatide is contraindicated in individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.
Notable Studies
The SURPASS clinical trials demonstrated significant reductions in HbA1c and body weight in patients with type 2 diabetes treated with Tirzepatide.
The SURMOUNT-1 trial showed that Tirzepatide led to substantial weight loss in obese individuals without diabetes.
Dosage
For type 2 diabetes: Initiate at 2.5 mg subcutaneously once weekly for 4 weeks, then increase to 5 mg once weekly. The dose may be increased in 2.5 mg increments after at least 4 weeks at the current dose, up to a maximum of 15 mg once weekly.
External link
-
Tirzepatide – StatPearls – NCBI Bookshelf
-
Tirzepatide: Uses, Interactions, Mechanism of Action – DrugBank
-
Tirzepatide: Uses, Dosage, Side Effects & Warnings – Drugs.com
-
Tirzepatide – Wikipedia
-
Mounjaro, Zepbound (tirzepatide) dosing, indications … – Medscape